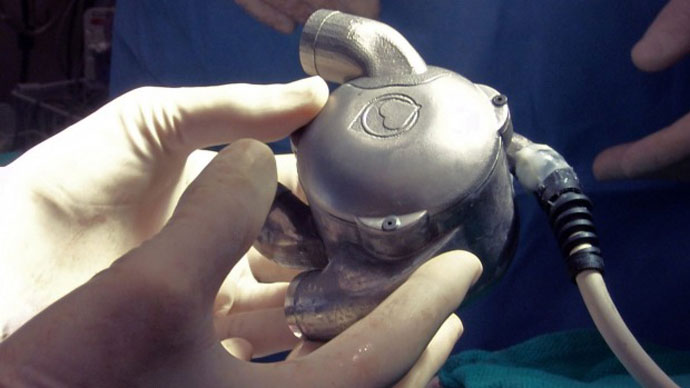
Pulseless Bionic Heart Could Be Ready For Human Trials Within 3 Years
A groundbreaking device known as BiVACOR could soon replace artificial hearts and organ donations. The world’s first bionic heart, designed by engineer Dr. Daniel Timms of Brisbane, Australia, was recently transplanted into a live sheep, and could be ready for human trials as early as 2018.
The bionic heart was first designed in 2001, when Timms was studying at the Queensland University of Technology, according to the Brisbane Times. Unlike a typical artificial heart, which uses a special sac to pump blood throughout the body with an artificial pulse, the BiVACOR has no pulse at all. Instead, it uses a small bladed disk, which spins at 2,000 revolutions per minute to pump blood throughout the body.
The BiVACOR heart could function for as much as 10 years, Timms told the Times.
Why so long? Because of the lack of wear and tear on the heart. As Timms points out in a video posted by crowdfunding organization The Common Good, the traditional sacs used to pump blood through artificial hearts undergo so much strain that they often break. Timms circumvented this by using mag-lev technology — basically, making the device’s components float. The bionic heart uses magnetic levitation to keep its parts from touching, which reduces deterioration significantly and could allow the heart to last for 10 years without wearing out. Traditional artificial hearts only last for about two years.
After the bionic heart was implanted into the sheep in January, Professor John Fraser of Prince Charles Hospital said it was “happy and healthy.”
However, at least 5 million Australian dollars are needed to take the heart to clinical trials for humans, which is where The Common Good comes in. The organization, which is an initiative of the Prince Charles Hospital, uses crowdfunding to support medical discoveries, and Timms’s team is hoping that the BiVACOR will receive enough funding that they can perfect the heart and its functions (such as making sure blood doesn’t clot) as well as test it on human patients.
We’ve now shown that the device works. This idea is viable. Now it’s a matter of making it robust and reliable so that it works in a patient,” Timms told the Brisbane Times. “The time frame is three to five years before it could be ready for humans. We need to test it for a year to confirm its safety and regulatory properties before we implant it in a patient.
Proving the concept was the first real hurdle. There are many to go from here but we’re confident we have the collaborative team to take it to that next level.”